^Radioactivity
^Radioactivity
1. A heavy unstable nucleus (e.g. Uranium, polonium, radium, thorium, actinium, etc.) disintegrates itself naturally, spontaneously & randomly without being forced by any external agent to do so until it acquires stability.
2. The disintegration is independent of all physical and chemical conditions and so it can neither be accelerated nor retarded.
3. The disintegration is random. It is purely a matter of chance for any atom to disintegrate first. It is not possible to predict whether a particular nucleus will decay in a given time interval.
4. The activity (or rate of disintegration, A or R) of a radioactive sample at any instant is directly proportional to the number of undecayed nuclei present in the sample at that instant.
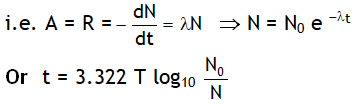
Here λ = disintegration constant or decay constant. & N0 = no. of the atoms present initially i.e. at t = 0.
From above result we can say
- The number of active nuclei in a radioactive sample decreases exponentially with time.
- The disintegration is fast in the beginning but becomes slower and with the passage of time.
- Irrespective of its nature a radioactive sample will take infinitively long time to disintegrate complete.
- The larger the value of decay constant l the higher is the rate of disintegration.
5. Half life (T): ![]()
6. Fraction ‘f’ of substance left undecayed after ‘n’ half lives is given by: ![]()
7. Mean life (τ): ![]()
8. Decay constant (λ) is the reciprocal of time for which ![]()
9. λ = 0 for a stable element (e.g. Pb).
10. (a) 1 Bacqueral (Bq) = 1 d.p.s.
(b) 1 Curie (rd) = 3.7 x 1010 d.p.s.
(c) 1 Rutherford (Rd) = 106 d.p.s.
Here d.p.s. = disintegrations per second.
