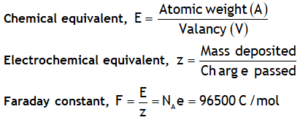^Cu voltameter
Cu voltameter
It consists of a glass vessel containing an aqueous solution of CuSO4 as electrolyte & two copper rods as electrodes. Copper sulphate in aqueous solution dissociated as ![]() .
.
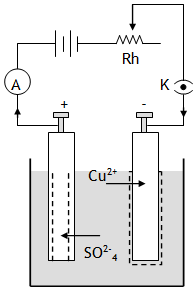
Due to the applied p.d. the SO2-4 ions drift towards the anode & the Cu2+ ions drift towards the cathode.
The SO2-4 ions on reaching the anode react with Cu atoms of anode to form CuSO4. i.e.
Cu + SO2-4 → Cu2+ SO2-4 + 2 e–
Also the oxidation reaction at the anode is
Cu → Cu2+ + 2e–
These Cu2+ ions dissolve into the solution, while the electrons so released flow towards the positive terminal of the battery via the external circuit. The Cu2+ ions on reaching the cathode get neutralized by the electrons flowing in from the negative terminal of the battery i.e. reduction occurs at the anode, Cu2+ + 2e– → Cu.
The net effect of electrolysis is that one copper atom is deposited at the cathode for each pair of electrons flowing through the connecting wires, thus copper is dissolved from the anode and deposited at the cathode in such a way that the concentration of CuSO4 in the solution remains constant & there is no accumulation of charge any where.