^Important masses
^Important masses

mn > mp ( slightly) mp > > me , mp » 1836 me

mn > mp ( slightly) mp > > me , mp » 1836 me
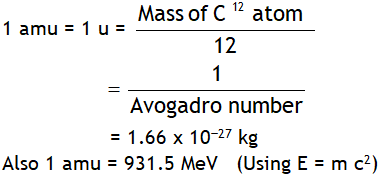
^Bohr correspondence principle
According to this principle the quantum theory must give same result as classical theory in the appropriate classical limit.
^Limitations of Bohr’s theory
(i) Quantum (to explain the existence of stationary orbits) &
(ii) Classical (for motion of electrons in the orbits). These two theories essentially oppose each other.
^Newton’s law of motion
Isaac Newton (1643 – 1727) published Principia Mathematical in 1687. In this work, he proposed three “laws” of motion.


^Rydberg’s formula
Let ‘E’ be the energy & λ be the wavelength of the photon released when an electron jumps from a higher quantum state of principal quantum number n2 to a lower quantum state having principal quantum number n1, then
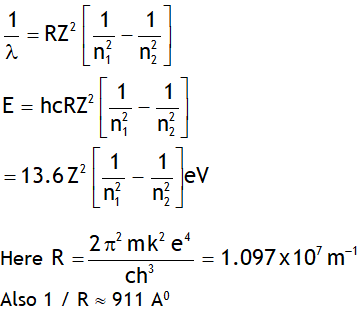
Note Rydberg’s constant depends on mass of
Electron, thus it is not a universal constant.
In deriving the above value the nucleus is assumed to be at rest. However if nucleus is not assumed stationary then the Rydberg constant depends on both mass of electron & nucleus & is given by
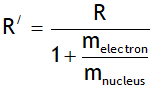
^Bohr’s frequency condition
Energy is emitted only when an electron exited to the higher states jumps back to lower states. The energy emitted is described by the relation
h f = E1 – E2
Ionization energy = +13.6 eV Z2
^Energy level diagram
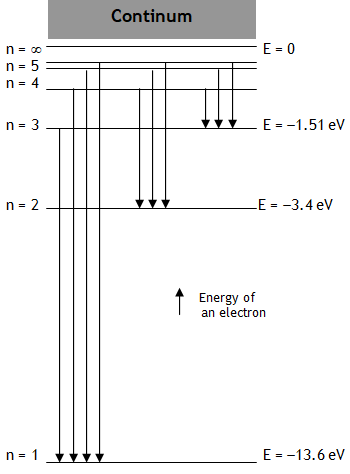
With the increase in the value of principle quantum number n
(a) r, L, T, U & E all increase while
(b) v, K, & w all decrease.
^Energy in nth orbit
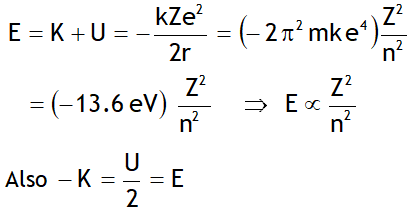
Here the -ve sign of energy shows that electron is bound to the nucleus & is not free.
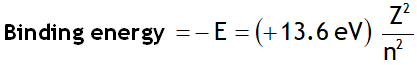
The binding energy of the electron in the ground state of the H-atom is called Rydberg. i.e.
1Rydberg = 13.6 eV