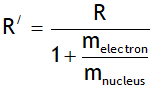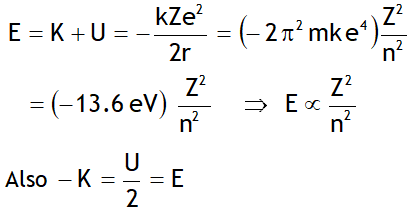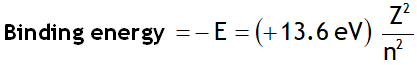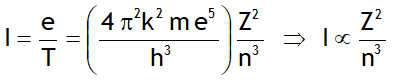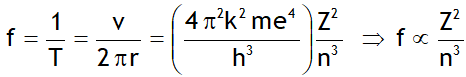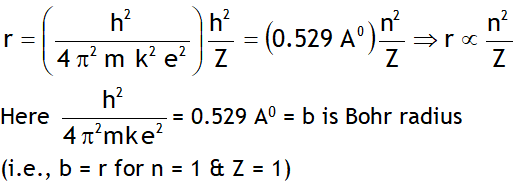^Rydberg’s formula
^Rydberg’s formula
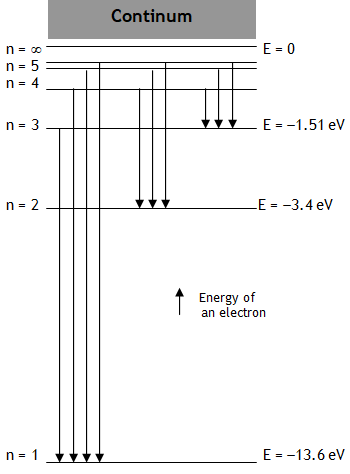
Let ‘E’ be the energy & λ be the wavelength of the photon released when an electron jumps from a higher quantum state of principal quantum number n2 to a lower quantum state having principal quantum number n1, then
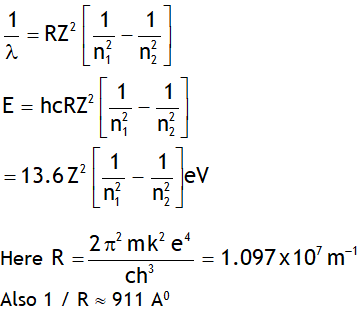
Note Rydberg’s constant depends on mass of
Electron, thus it is not a universal constant.
In deriving the above value the nucleus is assumed to be at rest. However if nucleus is not assumed stationary then the Rydberg constant depends on both mass of electron & nucleus & is given by