^Zener diode
^Zener diode
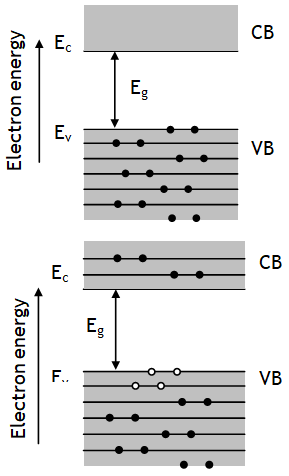
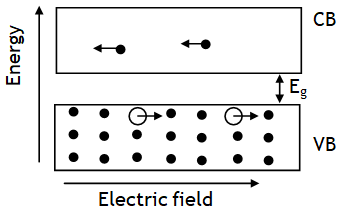
It is a specially designed junction diode, which can operate in the reverse breakdown voltage region continuously without being damaged. In forward bias behaves like ordinary diode. Zener diode does not gets damaged at breakdown voltage, but it does so at some higher reverse voltage, known as its ‘burn out value’. The magnitude of zener voltage VZ can be decreased by increasing doping level in p and n type materials of zener diode.
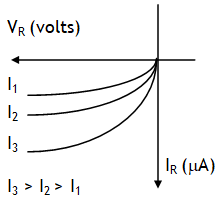
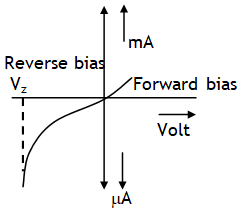
I – V characteristics of a Zener diode are as shown. For a voltage more than zener breakdown voltage current through diode increases without increase in voltage across it. This feature is exploited to provide voltage stabilization across a circuit.
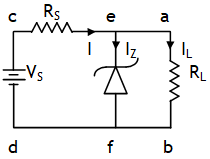
Suppose a load RL connected between a & b is connected to a fluctuating dc voltage supply Vs between c & d. Let the load can’t tolerate a voltage VL but the supply Vs can be more than VL. To protect the load from any increase voltage a zener diode of breaking voltage VZ = VL is connected to the input supply with a safety resistor RS in the input loop.
If at any time VZ increases than VL the zener diode works in the breakdown region increasing the current through it without increasing the voltage across it & thus the load always remains protected.