^Mass defect
^Mass defect
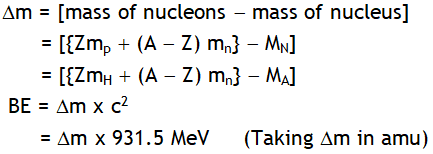
^Nuclear Force
It binds the nucleons together protons and neutrons together in the nucleus of an atom against the repulsion of positively charged protons. It is a is short range force & believed to be due to the exchange of pions (also called π – mesons) between the nucleons. It is the strongest (as Fg: F e: Fn = 1: 1036: 1038) of the fundamental forces. Also it is Short range, Non central, Saturated, Attractive as well as repulsive.
^Density of nucleus
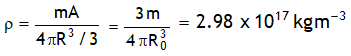
Nuclear density is independent of Mass number.
^Nucleus
Scattering experiments using fast electrons (instead of α – particles) as projectiles on targets of various elements, the sizes of nuclei of various elements have been accurately measured to be R = R0 A1/3
Here R0 = 1.2 × 10 -15 m known as nuclear unit.
^Neutrons
1. Neutron (discovered by James Chadwick in 1932) is an elementary particle present in the nuclei of all elements except hydrogen.
2. The mass of a neutron is slightly more than that of a proton & is now known to a high degree of accuracy.
It is mn = 1.00866 u = 1.6749×10 –27 kg
3. Neutron has no charge. Being neutral
(a) doesn’t interact with electrons & & doesn’t ionize the gas & hence doesn’t produce any track in the Wilson Cloud chamber.
(b) are not repelled or attracted by the nucleus and the electrons of an atom & consequently can easily penetrate heavy nuclei and induce nuclear reactions.
4. Inside a nucleus, a neutron is stable. But outside a nucleus, it is unstable. A free neutron spontaneously decays into a proton, electron and antineutron (an elementary particle with zero charge and zero rest mass) with a mean life of about 1000 s.
![]()
^Nucleus of an atom
Large angle scattering of α-particles by thin metal foils in Rutherford’s experiment revealed
1. Nuclear size is found to be of the order of 10–14 m whereas the diameter of an atom is of the order of 10–10 Hence most of the atom is empty or nucleus of an atom is a very tiny central region.
2. Charge of a nucleus of atomic number Z = +
3. More than 99.9% of the mass of an atom is concentrated in the nucleus.
^A horizontal pull on three blocks
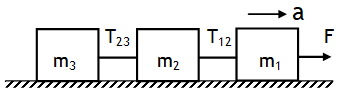
Using NSL, we can write
System m1: m1 a = F – T12
System m2: m2 a = T12 – T23
System m3: m3 a = T23
System total mass: (m1 + m2 + m3) a = F
^Facts
^Bragg’s law
If d is the spacing of the crystal planes, then diffraction of X-rays takes place according to the Bragg law : d sin θ = nλ
Here n = 1, 2, 3, _ _ _ _ _ _ _ _ & ‘θ’ is the angle of diffraction or grazing angle.
^Intensity & penetration of X–rays